

Eurofins Scientific is a leading international group of laboratories with revenues of 3 billion per year, 45,000 employees in 650 laboratories in 45 countries around the world. Our laboratories provide a unique range of research services for pharmaceuticals, food, industry and consumer goods.


The Institute is the basic research and development institution in scientific research and development work leading to new technical and organizational solutions in the field of labor protection in the field of occupational safety, health and ergonomics, as well as performing other tasks particularly important for achieving the goals of the social and economic policy of the state in the field.


Office dealing with the authorization of medicinal products, placing on the market and putting into use of medical devices, clinical trials, including veterinary clinical trials - Pharmaceutical law on medical devices.


The CE marking / marking (Conformité Européenne) placed on the product is the manufacturer's declaration that the marked product meets the requirements of the directives. The "New Approach" of the European Union (EU).


The main goal of the Medical Diagnostic Laboratories of NIZP-PZH is to provide you with a high standard of services and reliable results of laboratory tests.


Specjalistyczne Laboratorium Badawcze Skin Lab International Sp. z o. o. is a dynamically developing research center that carries out a comprehensive package of research and analysis. He specializes in dermatological, application and implementation research for the cosmetics and chemical industry, and in specialized research for the light industry.
How medical masks differ between one another?

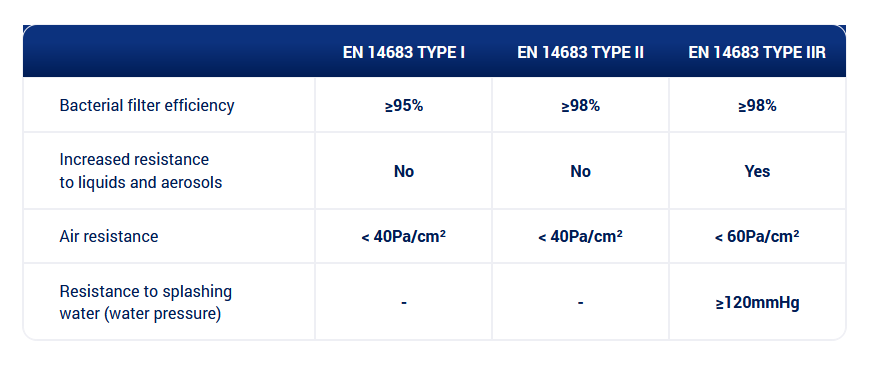
Depending on the purpose, there are the following types of medical masks: type I, type II and type II R. Each type differs with respect to material filtration efficiency (BFE), resistance to liquids and resistance of exhaled air which affects the comfort of use.
What are medical masks intended for?

The main purpose of medical masks is to protect the environment of the medical staff against viruses. This function is ensured by means of special filtering material which stops the droplets of the exhaled air. Medical mask should fit tightly to the face so as to protect its user (to a small extent). The II R medical mask additionally retains fluids and liquids.
Who should use medical masks?

Medical masks should be used by medical staff in any space in which direct or indirect contact with the patient may occur. This way, the risk of transmission of viruses by droplets is limited to a minimum.
How tight are medical masks?

Unfortunately, the medical mask is not completely tight, which is why it is so important that it is correctly worn so that it adheres closely to the face.
How long can a medical mask be worn?

After some time, the filtering material in the medical mask becomes damp and no longer provide protection. Therefore, medical masks are disposable products which should be changed when they become wet. Regardless of how wet the mask is, it should be replaced no later than 3–4 hours after wearing it.
What are the differences between medical masks?

Depending on their intended use, medical masks are divided into types: type I, type II and type II R . The individual types differ from each other in such elements as: the effectiveness of the filter material (BFE), resistance to soaking of liquids and the resistance of exhaled air, which affects the comfort of using the mask.
How to distinguish a safe, all-standard medical mask from a regular non-woven one? What should be verified before buying?

Currently, there are a number of “medical” masks on the market, often at a good price. Unfortunately, many of them do not meet any standards for medical products. The requirements for medical masks are described in the EN 14683 standard. They refer both to the product itself (mask) and to the manufacturing process.
Before buying, verify the following:
Does the package bear the EN 14683 marking and the CE mark?
Has the product been registered in the Office for Registration of Medicinal Products, Medical Devices and Biocides? (VAT on medical masks is 8% while VAT on non-medical masks is 23%)
Have the manufacturer conducted and is willing to present the results of microbiological tests performed by an approved laboratory?
Does the manufacturer have a document confirming that medical masks are manufactured in accordance with the ISO cleanroom standards required by law?

Headquarters in Poznań: